Affinity vs Avidity
Antibody basics
Antibodies are expressed on the surface of B cells, one of the major cell types involved in adaptive immunity. Because each B cell must express a unique antibody for an individual to mount an effective immune response, an almost infinite number of antibodies exists. Antibody diversity is achieved via three main mechanisms – V(D)J recombination, junctional diversification, and somatic hypermutation – with the latter producing higher affinity antibodies (affinity maturation) as the adaptive immune response unfolds. Affinity is one of two key properties that defines the overall strength of the antibody-antigen interaction. The other is avidity, which has a different meaning.
What is affinity?
Affinity, also known as binding affinity, is the strength of the interaction between the antigen-binding site (paratope) on an antibody and the epitope on an antigen. Critically, it refers to the strength of the individual bond between the paratope and epitope contact residues. Affinity is mediated by non-covalent interactions that include hydrogen bonds, electrostatic bonds, Van der Waals forces, and hydrophobic interactions and is defined by the equilibrium dissociation constant (KD). KD is the calculated ratio of the rate at which the antibody binds its target and koff is the rate at which the antibody-antigen complex dissociates. High affinity antibodies have a low KD and are characterized by rapid target recognition (high kon) and strong stability of the resultant antibody-antigen complex (low koff).
What is avidity?
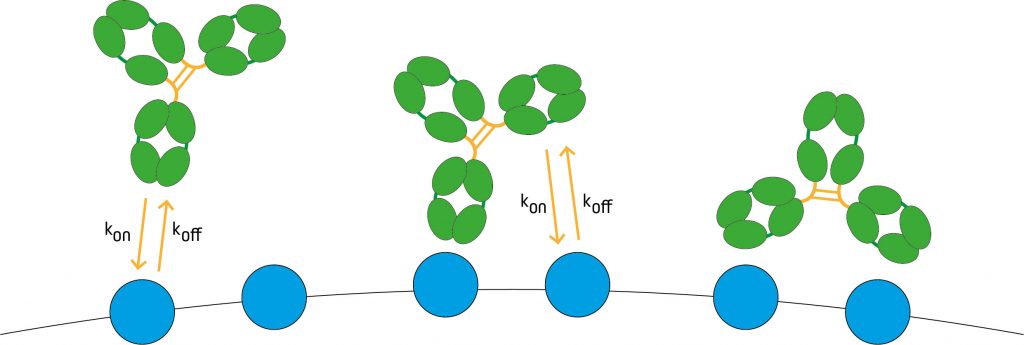
Avidity, also known as functional affinity, represents the overall strength of the antibody-antigen interaction and is influenced by three factors – the binding affinity (described above), valency, and the structural arrangement of the antibody and antigen in question. When discussing valency, it is important to remember that this refers not only to the number of antigen binding sites on the antibody, but also to the number of residues contributing to the paratope. To put this into context, while whole IgG molecules are bivalent, Fab fragments are monovalent, and naturally-occurring IgA and IgM have four and ten binding sites, respectively, the complementarity-determining regions (CDRs) of each antibody typically contain multiple residues involved in antigen binding; all of these interactions in combination contribute to the avidity.

The structural arrangement of the antibody-antigen complex can have a significant impact on avidity. Where experimental conditions (e.g., fluctuating temperature or pH) cause even a slight change in antibody or antigen conformation, this can impact results.
What other properties influence antibody performance?
In addition to affinity and avidity, two other fundamental properties of antibodies determine their performance. The first of these is specificity, which is how well the antibody discriminates between one antigen and another. Both high affinity and low affinity antibodies can share similar specificities; however, it is the B cells which produce high affinity antibodies that are favored for expansion by the immune system. The other property is selectivity, which describes how well an antibody binds its antigen within a heterogeneous mixture.
Experimental considerations
Antibody affinity, avidity, specificity, and selectivity can all be influenced by experimental conditions, highlighting the critical importance of antibody selection and assay optimization. Factors to consider when using antibodies for research include the antigen structure (e.g., whether it is native or denatured), the amount of antigen present in the sample, the antibody concentration, the buffer composition, and the incubation temperature and time, to name just a few. To avoid generating misleading data, it is advised that researchers purchase well-characterized antibodies from a trusted supplier and that they test these in their own model system before proceeding with hypothesis-driven research.
Jackson ImmunoResearch specializes in producing secondary antibodies for life science applications. Our portfolio includes antibodies conjugated to enzymes, fluorophores, and other biomolecules, all of which have been rigorously validated in-house, and we also offer minimal cross-reactivity (Min X) secondary antibodies that have been cross-adsorbed to minimize the risk of background signal.
References
Ref: https://www.nature.com/articles/s42003-020-01319-z

